History
GCRSR Chair and Vice Chair
-
2023 - Present:
-
2022 - 2023:
-
2018 - 2021:
-
2016 - 2017:
2014 - 2015:
2013:
-
Chair: Dr. Weida Tong, National Center for Toxicological Research (NCTR), Food and Drug Administration (FDA), USA
Co-Chair: Dr. DAS NEVES Carlos, European Food Safety Authority (EFSA), EU
-
Chair: Dr. Weida Tong, National Center for Toxicological Research (NCTR), Food and Drug Administration (FDA), USA
Vice Chair: Dr. Georges Kass, European Food Safety Authority (EFSA), EU
-
Chair: Dr. William Slikker Jr, National Center for Toxicological Research (NCTR), Food and Drug Administration (FDA), USA
Vice Chair: Dr. Marta Hugas, European Food Safety Authority (EFSA), EU
-
Chair: Dr. William Slikker Jr, National Center for Toxicological Research (NCTR), Food and Drug Administration (FDA), USA
Vice Chair: Dr. Carlos Chile, The National Administration of Drugs, Foods and Medical Devices (ANMAT), Argentina
Chair: Dr. William Slikker Jr, National Center for Toxicological Research (NCTR), Food and Drug Administration (FDA), USA
Vice Chair: Dr. Marion Healy, Food Standards Australia New Zealand (FSANZ), Australia
Chair: Dr. William Slikker Jr, National Center for Toxicological Research (NCTR), Food and Drug Administration (FDA), USA
Important Events
2011
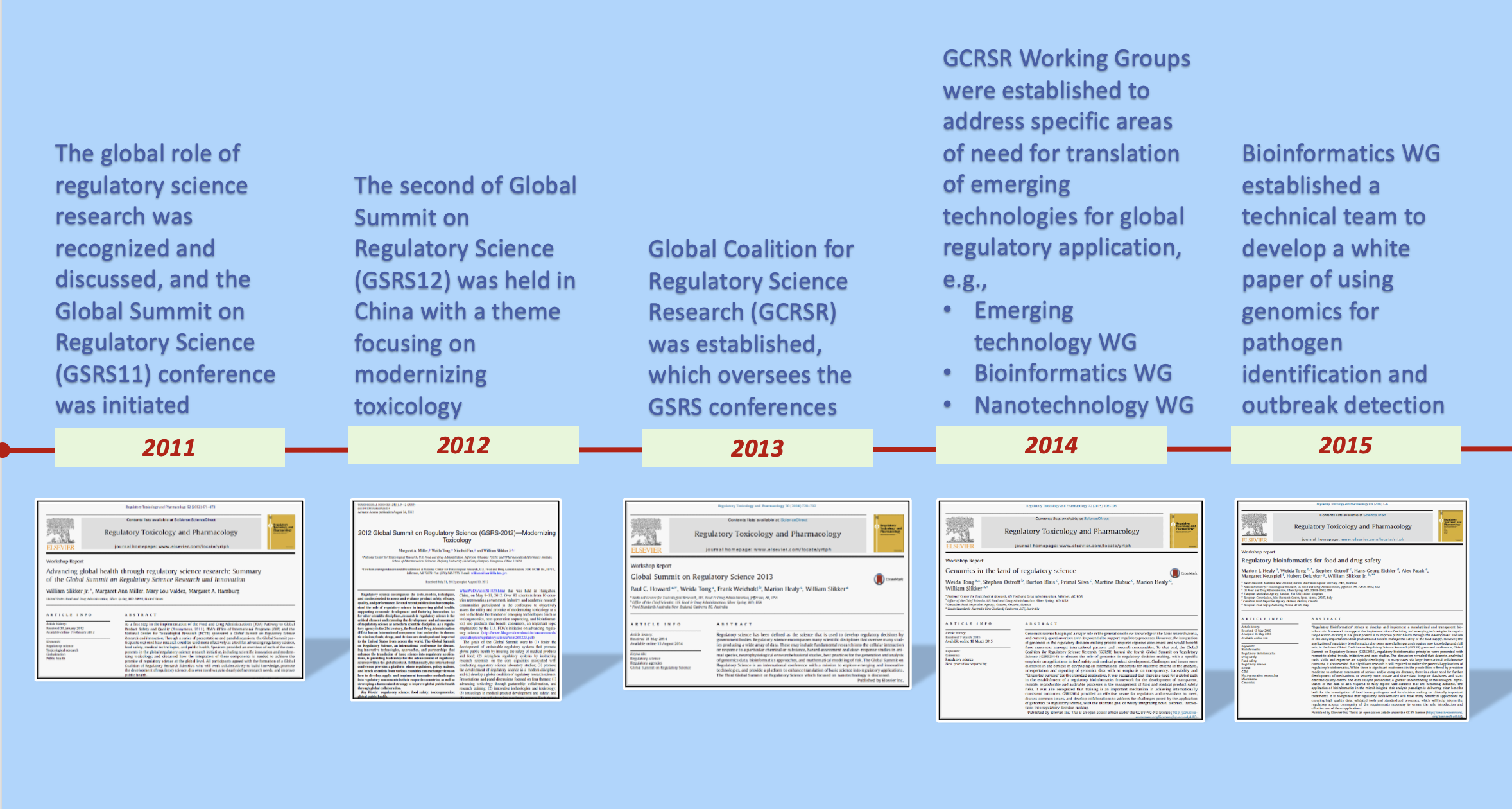
• The global role of regulatory science research was recognized and discussed, and the Global Summit on Regulatory Science (GSRS11) conference was initiated.
2012
• The second of Global Summit on Regulatory Science (GSRS12) was held in China with a theme focusing on modernizing toxicology.
2013
• Global Coalition for Regulatory Science Research (GCRSR) was established, which oversees the GSRS conferences.
2014
• GCRSR Working Groups were established to address specific areas of need for translation of emerging technologies for global regulatory application, e.g.,
• Emerging technology WG
• Bioinformatics WG
• Nanotechnology WG.
2015
• Bioinformatics WG established a technical team to develop a white paper of using genomics for pathogen identification and outbreak detection.
2016
• Nanotechnology WG developed the white paper on advancing Nanotechnology Standards in regulatory application.
2017
• The Bioinformatics WG technical team developed/published the best practices document for microbial genomics in application for regulatory food safety.
• The Cross-Training WG was established.
2018
• The Shared Regulatory Research Priorities WG was established.
2011

The global role of regulatory science research was recognized and discussed, and the Global Summit on Regulatory Science (GSRS11) conference was initiated.

2012

The second of Global Summit on Regulatory Science (GSRS12) was held in China with a theme focusing on modernizing toxicology.

2013

Global Coalition for Regulatory Science Research (GCRSR) was established, which oversees the GSRS conferences.

2014

GCRSR Working Groups were established to address specific areas of need for translation of emerging technologies for global regulatory application, e.g.,
Emerging technology WG
Bioinformatics WG
Nanotechnology WG.

2015

Bioinformatics WG established a technical team to develop a white paper of using genomics for pathogen identification and outbreak detection.

2016

Nanotechnology WG developed the white paper on advancing Nanotechnology Standards in regulatory application.

2017

The Bioinformatics WG technical team developed/published the best practices document for microbial genomics in application for regulatory food safety.
The Cross-Training WG was established.

2018

The Shared Regulatory Research Priorities WG was established.

2019


2020


2021


2022


Contact Us: gsrsconferences@fda.hhs.gov
Copyright © 2024 GCRSR