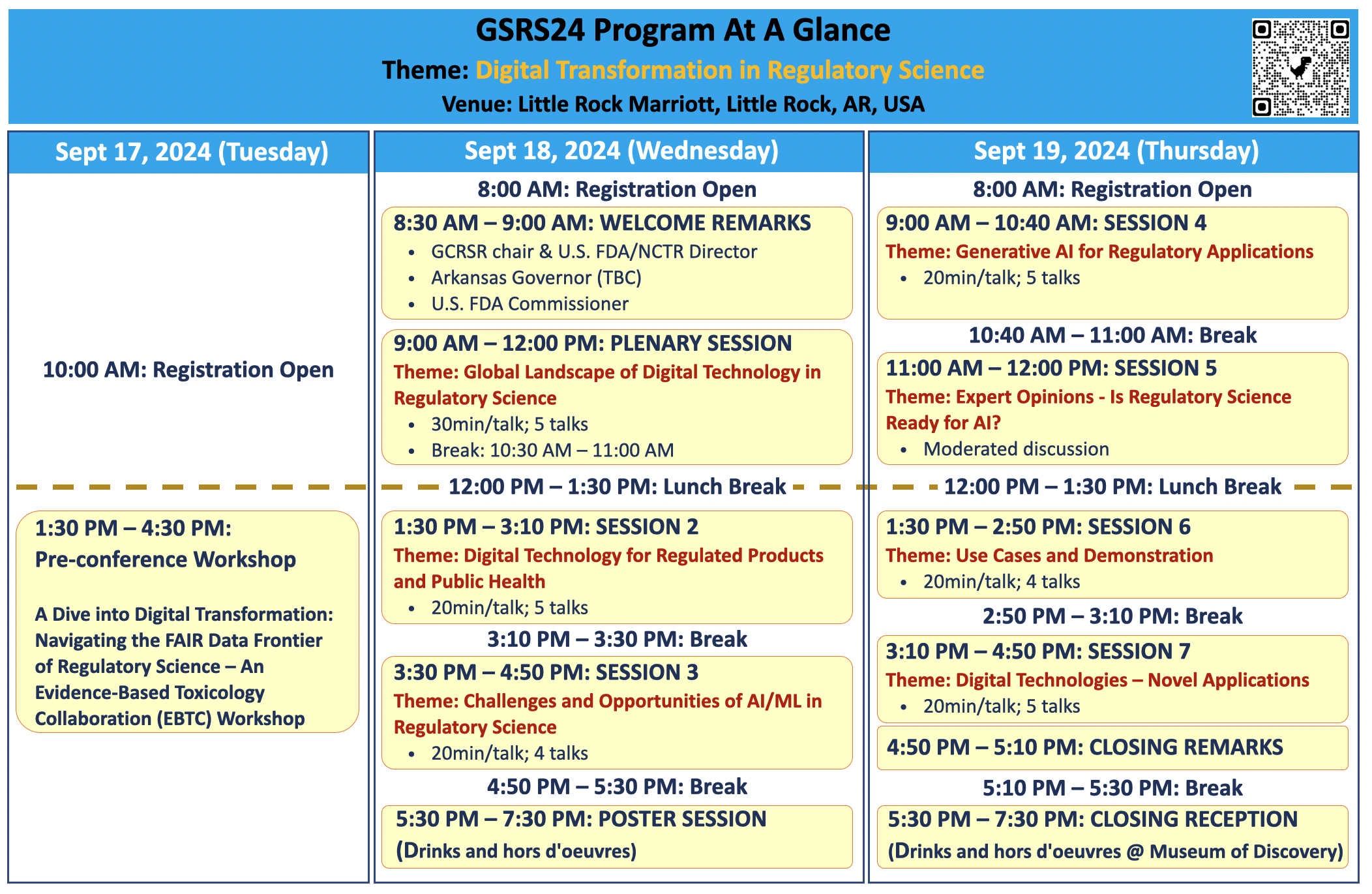
program
All times are in Central Standard Time (GMT-6)
All times are in Central Standard Time (GMT-6)
A dive into digital transformation: navigating the FAIR data frontier of regulatory science: An EBTC Workshop
Tuesday, September 17, 2024, 1:30-4:30 PM
Moderators:
Katya Tsaioun and Paul Whaley
Evidence-based Toxicology Collaboration at Johns Hopkins Bloomberg School of Public Health www.EBTox.org
Objective:
Levelling participants' understanding of FAIR data for regulatory science.
Focus:
Practical understanding and application of FAIR data principles.
• In silico-driven drug discovery and repurposing
• De-risk of drug applications via computational methods
• Digital technology for improved diagnosis and therapeutic options for rare disease
• Digital regulatory science to address the opioid crisis, and other significantly regulatory concerns such as CBD, Nitrosamines and PFAS
Aim:
Enhance participant engagement and contribution at GSRS Conference.
Emphasis:
Understanding FAIR data's role in research, risk assessment, and regulatory decision-making.
Structure:
1. Presentations to prime detailed breakout discussion.
• Open science and FAIR data basics.
• Importance of data standards to regulators at the NIH.
• Case study on FAIRness: Drug-induced liver injury.
2. Presentations to prime detailed breakout discussion.
• Hands-on analysis of example studies' data accessibility.
• Discussion on open data features and issues.
3. Presentations to prime detailed breakout discussion.
• Reacting to bold statements on digital transformation.
• Moderated to maximize participant contribution.
Speakers:
1. Paul Whaley, Co-Chair Open Science Working Group, EBTC.
2. TBD, Director Office of Data Science, NIH (TBC).
3. Katya Tsaioun, Executive Director, EBTC.
Innovation:
1. Interactive workshop to increase engagement of the audience and reinforce the concepts.
2. Engaging participants with varied backgrounds to share experiences.
3. Moderation to ensure productive discussion and networking.
Benefits for Participants:
1. Interactive session to demystify FAIR data and open science.
2. Practical exercise to grasp FAIR data usage in research.
3. Networking.
All times are in Central Standard Time (GMT-6)
Day 1—Wednesday, September 18
08:30-09:00
Welcome Remarks by GCRSR Chair Dr. Weida Tong and U.S. FDA/NCTR Director Dr. Tucker Patterson
• The Honorable Sarah Huckabee Sanders, Governor of Arkansas (TBC)
• Dr. Robert M. Califf, U.S. FDA Commissioner
9:00-12:00 PM SESSION 1 (PLENARY SESSION): GLOBAL LANDSCAPE OF DIGITAL TECHNOLOGY IN REGULATORY SCIENCE
Co-Chairs: Dr. Tucker Patterson (U.S. FDA); Ms. Elaine Johanson (U.S. FDA)
9:00-9:30
Transforming the Future of Regulatory Science
Ms. Elaine Johanson, Director, Health Informatics Staff, Office of Data, Analytics, & Research (ODAR), U.S. Food and Drug Administration (FDA), USA
9:30-10:00
Advancing Risk Assessments through FAIR Knowledge Exchange: The RAKIP Initiative
Mr. Matthias Filter, Head of Study Centre for Food Chain Modelling and Artificial Intelligence, German Federal Institute for Risk Assessment (BfR), Germany
10:00-10:30
Break
10:30-11:00
Modernizing regulatory practices through digital tools and technologies: Saudi Food and Drug Authority Experience
Dr. Adel Alrwisan, Executive Director of Research and Studies Department, Saudi Food & Drug Authority (SFDA), Saudi Arabia
11:00-11:30
Digital Transformation and Use of AI Tools: ANVISA Experience
Mr. Anderson da Mota Ribeiro, Data Analysis Manager, Brazilian Health Regulatory Agency (ANVISA), Brazil
11:30-12:00
When Culture Devours Strategy: Navigating the Cultural Challenges of AI Implementation in the Public Sector
Mr. Michael Renaudin, Lead Swissmedic 4.0, Swissmedic, Switzerland
12:00-1:30
Lunch Break
1:30-3:10 PM SESSION 2: DIGITAL TECHNOLOGY FOR REGULATED PRODUCTS AND PUBLIC HEALTH
Co-Chairs: Dr. Bill Slikker (Former Director of U.S. FDA/NCTR) ; Dr. Yoko Hirabayashi (National Institute of Health Sciences, Japan)
1:30-1:50
Trustworthy AI for Public Health Decisions Making: Is There Consensus on Evaluating & Documenting AI Tools Used by Authorities
Dr. Claudius Griesinger, Member of the Leadership Team of the JRC’s project portfolio on “Innovation in Life and Health Sciences,” European Commission Joint Research Centre (EC-JRC), EU
1:50-2:10
Leveraging Reader Studies for Digital Pathology
Dr. Kim Blenman, Assistant Professor, Department of Internal Medicine and Department of Computer Science, Yale University, USA
2:10-2:30
Harnessing the Value of Digital Health Technologies in Clinical Development
Dr. Jie Shen, Director of Digital Science, AbbVie, USA
2:30-2:50
AllerCatPro 3.0 - Protein Allergenicity Prediction with 3D Structure Features
Dr. Minh Nguyen, Principal Scientist I at Bioinformatics Institute, A*STAR - Agency for Science, Technology and Research, Singapore
2:50-3:10
Current Status and Challenges for the Use of AI in the Pharmacovigilance Field in Japan
Dr. Noriaki Arakawa, Section Chief of Division of Medicinal Safety Science, National Institute of Health Sciences (NIHS), Japan
3:10-3:30
Break
3:30-4:50 PM SESSION 3: CHALLENGES AND OPPORTUNITIES OF AI/ML IN REGULATORY SCIENCE
Co-Chairs: Dr. Maurice Whelan (European Commission-JRC); Dr. Suzanne Fitzpatrick (U.S. FDA)
3:30-3:50
The Race for Regulation: Overview of Regulatory Efforts to Guide AI/ML Application and Acceleration
Mr. Cesare Furlanello, Director of LIGHT Center, Italy
3:50-4:10
AI at the European Food Safety Authority: Our Journey from Innovation to Implementation
Dr. Didier Verloo, Head of Knowledge Innovation and Partnership Management Unit (KNOW), European Food Safety Authority (EFSA), Italy
4:10-4:30
Utilization of Machine Learning on the Classification of Silicone Oil Droplets and Protein Particles in Biopharmaceutical Products
Dr. Hiroko Shibata, Section Chief of Division of Biological Chemistry and Biologicals, National Institute of Health Sciences (NIHS), Japan
4:30-4:50
Top 10 AI/ML Mistakes and Villains
Dr. Russ Wolfinger, Director of Scientific Discovery and Genomics, JMP Statistical Discovery, SAS Institute Inc, USA
5:30-7:30 PM Poster Presentations (Drinks and hors d'oeuvres)
All times are in Central Standard Time (GMT-6)
Day 2—Thursday, September 19
9:00-10:40 AM SESSION 4 | GENERATIVE AI FOR REGULATORY APPLICATIONS
Co-Chairs: Dr. Kern Rei Chng (Singapore Food Agency); Dr. Dongying Li (U.S. FDA)
9:00-9:20
LLM Task Force Review: Lessons Learned and Future Challenges
Mr. Alexander Horst, Data Scientist, Swissmedic 4.0, Swissmedic, Switzerland
9:20-9:40
Harnessing Generative AI for Sense-Making of Foodborne Outbreak Investigation Reports
Mr. Benjamin ER, Team Lead of Food Safety Analytics & Epidemiology, Singapore Food Agency (SFA), Singapore
9:40-10:00
Presentation Title (TBD)
Speaker from either EMA or HC (TBD)
10:00-10:20
AskFDALabel: Enhancing Drug Reviewers’ Experience with Large Language Model in Daily Missions
Dr. Leihong Wu, Research Scientist, Division of Bioinformatics and Biostatistics, National Center for Toxicological Research, US FDA, USA
10:20-10:40
Collaborative Innovation: Unveiling a Use Case from Our Collabathon
Dr. Nicolas Perez, Data Scientist, Swissmedic, Switzerland
10:40-11:00
Break
11:00-12:00 PM SESSION 5| EXPERT OPINIONS - IS REGULATORY SCIENCE READY FOR AI?
Moderator:
Dr. Weida Tong, Director of Division of Bioinformatics and Biostatistics, National Center for Toxicological Research, US FDA, USA
Panel:
Dr. Thomas Hartung, Director of the Center for Alternatives to Animal Testing (CAAT), Johns Hopkins University, USA
Dr. Maurice Whelan, Deputy Director for Health and Food, Head of the Systems Toxicology Unit, European Commission Joint Research Centre (EC-JRC), EU
12:00-1:30
Break
1:30-2:50 PM SESSION 6| USE CASES AND DEMONSTRATION
Co-Chairs: Ms. Laila Sofia Mouawad (Brazilian Health Regulatory Agency); Mr. Michael Renaudin (Swissmedic)
1:30-1:50
Navigating Innovation in a Regulatory Agency – A Management Perspective
Dr. Philippe Girard, Vice Director, Head of Medicinal product licences and surveillance, Swissmedic, Switzerland
1:50-2:10
Introducing TKPlate - Food Safety Without Animal Testing?
Dr. Didier Verloo, Head of Knowledge Innovation and Partnership Management Unit (KNOW), European Food Safety Authority (EFSA), Italy
2:10-2:30
Automating the Surveillance of Products on the Internet: EPINET Tool
Mrs. Mariana Adelheit Von Collani, Enforcement Advisor, Brazilian Health Regulatory Agency (ANVISA), Brazil
2:30-2:50
Streamline Clinical Review of Drug Application with a Widely Used Tool by Global Regulatory Agencies
Dr. Wenjun Bao, Chief Scientist and Director of Advanced Analytics R&D, JMP Statistical Discovery, SAS Institute Inc, USA
2:50-3:10
Break
3:10-4:50 PM SESSION 7 | DIGITAL TECHNOLOGIES – NOVEL APPLICATION
Co-Chairs: Dr. Tammy Collins (Burroughs Wellcome Fund); Dr. Catherine Carrillo (Canadian Food Inspection Agency)
3:10-3:30
Bridging Hybrid AI and Digital Measures: Innovating from Preclinical Research to Clinical Trials
Dr. Szczepan Baran, Chief Scientific Officer, VeriSIM Life, USA
3:30-3:50
Data Science and Machine Learning in Microbial Omics: Standardization, Applications, and Challenges
Dr. Julie Chih-yu Chen, Head of Data Sciences, Bioinformatics Section, National Microbiology Laboratory Branch, Public Health Agency of Canada (PHAC), Canada
3:50-4:10
Working Better Together – From Data Harmonization to Data Integration
Dr. William Hsiao, Associate Professor, Simon Fraser University (SFU), Canada
4:10-4:30
ML/AL Based Allergenicity Prediction of Novel Food
Dr. Norimasa Tamehiro, Section Chief of Division of Biochemistry, National Institute of Health Sciences (NIHS), Japan
4:30-4:50
Application of Deep Learning Convolutional Neural Networks to Identify Gastric Squamous Cell Carcinoma in Mice
Dr. Zhi Lin, Deputy Director of Pathology Department of National Center for safety Evaluation of Drugs, National Institutes for Food and Drug Control (NIFDC), China
4:50-5:10 PM Announcement of GSRS25 and Closing Remarks
5:30-7:30 PM
Closing Reception (Drinks and hors d’oeuvres at Museum of Discovery)
15:15-15:25
Opening Remarks
Co-chairs
15:25-15:45
Innovation in Regulatory Science Awards at Burroughs Wellcome Fund —Preview of the Latest Technologies and Progress Towards Equitable Clinical Outcomes
Q&A
APRIL Template
Support Center
Standard Support Enquiries
standard@april.com
Premium Support Enquiries
premium@april.com